HOME > English
About us
The bacterial community in the oral cavity is one of most complex mixtures of known bacteria. We know that human oral cavity is a highly dynamic ecosystem that supports the growth of a tremendous number of very diverse organisms. In fact, there are roughly ten million organisms per milliliter of saliva and a billion per milligram of dental plaque. The organisms present in the oral cavity, mostly bacteria and a few fungi, are there because they are shed from the hard and soft tissues of both oral cavity and nasopharynx, and they multiply in retained pools of saliva. The use of microbiological techniques, coupled with sophisticated and sensitive technologies in molecular biology, has helped us to further gain a deeper appreciation of the oral microbiota diversity. Recent estimates place the number of different species of bacteria in the oral cavity at around 600~700 per milligram of dental plaque. Over half of these have not been cultivated and more species are continuing to be discovered.
Research into the genetics, physiology, and biochemistry of the microbiota have shown that the normal colonizers are critical components of oral health and, thus, has led to a new understanding of its importance in oral ecology and in the development of diseases. Periodontal diseases are a complex chronic inflammatory condition caused by subgingival infection with oral bacteria and are risk factors towards developing several systemic diseases. The focus of our studies is the importance of microbial interaction in the induction of commensal bacterial-pathogenicity found orally and in the intestine.
Link: Nihon University School of Dentistry
Staff

Staff
- Professor
- Kuniyasu Ochiai, D.V.M., Ph.D
- ochiai.kuniyasu@nihon-u.ac.jp
- Associate Professor
- Kenichi Imai, D.D.S., Ph.D
- imai.kenichi@nihon-u.ac.jp
- Lecturer / Assistant Professor
- Hajime Tanaka, D.D.S., Ph.D
- tanaka.hajime@nihon-u.ac.jp
- Assistant Professor
- Muneaki Tamura, D.D.S., Ph.D
- tamura.muneaki@nihon-u.ac.jp
- Assistant Professor
- Noriaki Kamio, D.D.S., Ph.D
- kamio.noriaki@nihon-u.ac.jp
- Postdoctoral Fellow
- Marni E. Cueno, Ph.D
- marni.cueno@nihon-u.ac.jp
- SecretaryRika Hanabata
- Graduate StudentManabu Ohya
Research & Projects
- Kenichi Imai / Microbes-Host Interactions in Periodontal Diseases and Virus Infectious Diseases.
- Muneaki Tamura / Antimicobial gelight prevent not only oral diseases but also systemic diseases.
- Noriaki Kamio / Relationship between periodontal disease and metabolic syndrome.
- Marni E. Cueno / Plant-based approach towards stress and vaccines.
Kenichi Imai -Microbes-Host Interactions in Periodontal Diseases and Virus Infectious Diseases.
| 【Project 1】 | Elucidation of molecular mechanisms by which bacteria-viral interactions lead to progression of periodontal diseases. |
|---|
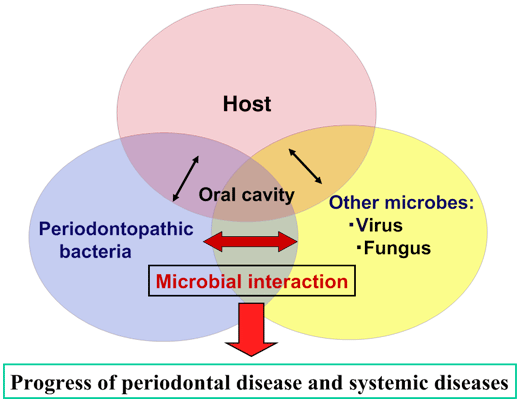
Periodontal disease, a complex chronic inflammatory disease caused by subgingival infection with oral bacteria such, as Porphyromonas gingivalis present in dental plaque, is found worldwide and is among the most prevalent microbial diseases in humans. It is marked by a breakdown of the periodontal tissue resulting from the interaction of specific anaerobic bacteria and host immune mechanisms. Severe periodontitis can result in loosening of teeth, occasional pain and discomfort, impaired mastication, and eventual tooth loss. Although the molecular mechanisms has not been established, recent studies have discovered a new way, viral infection is involved in progression of periodontal disease and periapical periodontitis. Our research focuses on how virus infection leads to progression of periodontal diseases(Fig.1). It is expected that enhancing our understanding of the pathogenesis of periodontal diseases involving viral infections will lead to new treatments and superior methods of prevention.
Fig 1 Periodontopathic bacteria interacts with host and other microbes
| 【Project 2】 | To investigate whether the concept of "periodontal medicine" is applicable to viral Infectious diseases. |
|---|
Porphyromonas gingivalis, a gram-negative anaerobic bacterium belonging to the phylum Bacteroidetes, is a major etiologic agent in the development and progression of periodontal disease in patients with adult chronic periodontitis. The bacteria produced an elaborate variety of virulence factors including LPS, fimbriae, gingipains, and short-chain fatty acids, such as butyric acid. During the past 10 years, accumulating evidence indicates that periodontal disease and P. gingivalis infection are risk factors for several systemic diseases, including diabetes, pre-term birth, heart disease and atherosclerosis. These associations show that P. gingivalis infection might be a causative factor in a number of systemic diseases. Recently, we observed that P. gingivalis could induce HIV-1 reactivation from latently infected HIV-1 cells via chromatin modification (Fig. 2) and presented a hypothetic model for the potential role of periodontal disease as a risk factor for HIV-1 activation in the infected individuals (1) (Fig. 3). In addition, we also found that P. gingivalis induces expression of the Epstein-Barr Virus lytic switch transactivator ZEBRA (2). Our observations suggest that periodontal diseases also affect viral infections, such as AIDS and EBV-related diseases. (Fig. 4)
- Ref;
- ⑴Current HIV Research (review), 2012, ⑵Biochimie, 2012.
Fig 2 Model of HIV-1 proviral latency and its reactivation
by P. gingivalis through chromatin remodeling.
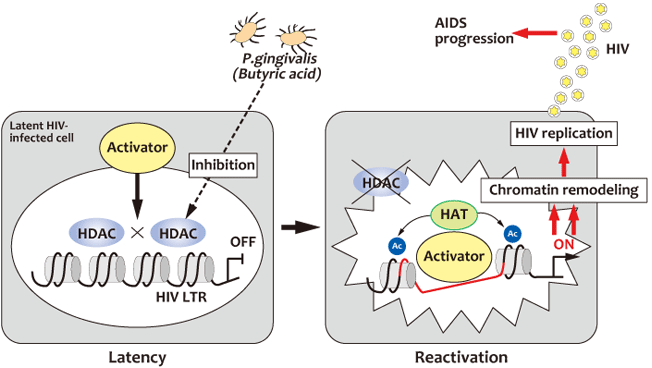
- Latent state:
- Closed chromatin structure at the latent HIV-1 LTR is promoted by the recruitment of
HDAC through negative transcription factors (e.g., AP-4, YY-1/ LBP-1, and NF-κB p50
homodimer),leading to HIV transcriptional silencing. - Productive state:
- P. gingivalis produces high concentrations of butyric acid causing disassociation and
inhibition of HDACs and subsequently recruitment of transcriptional activators and histone
acetyltransferases (e.g., CBP/ p300) exhibiting histone acetyltransferase activity to the
HIV-1 LTR promoter. Acetylation of local histones and loss of the above-mentioned
negative regulators together with HDAC proteins initiates transcription.
Fig 3 Possible causal link of periodontal diseases to AIDS progression.
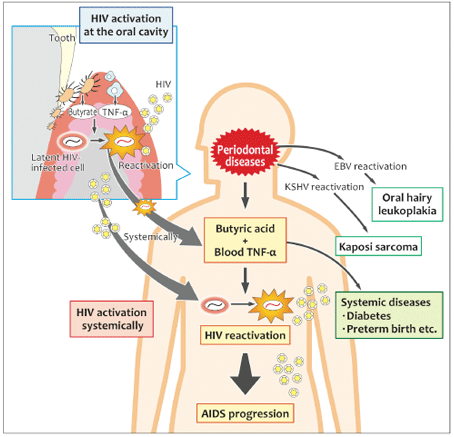
In individuals with AIDS, HIV-infected lymphocytes and monocytes are abundant at oral sites, including periodontal pockets, gingival tissues, and salivary glands. Direct interaction of these cells with periodontopathic bacteria and/or indirect interaction with soluble factors (eg, butyric acid and TNF-α) could induce local HIV-1 replication in the oral cavity. It is possible that cells in which viral transcription has been reactivated by a stimulus spread throughout the body via the blood. In addition, TNF-α concentrations are known to be elevated in individuals with periodontal disease, which suggests that periodontal disease is a trigger for local and systemic breakdown of latent infection and may be a risk factor for AIDS progression. Moreover, we have observed reactivation of EBV and Kaposi sarcoma-associated herpes virus (KSHV/HHV8) by periodontopathic bacteria. Thus,periodontal disease may be involved in the onset of AIDS-related pathological changes in oral hairy leukoplakia and Kaposi sarcoma.
Fig 4 Periodontal diseases and systemic diseases.
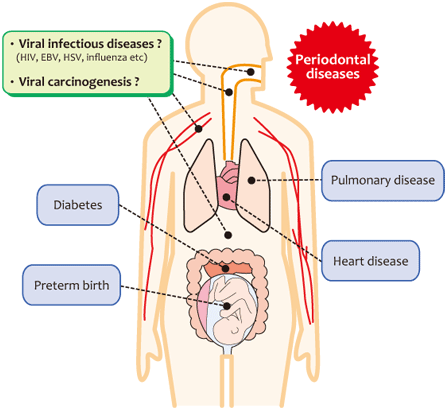
Mounting evidence supports the hypothesis that periodontal diseases are a risk factor for several systemic diseases. Our observations suggest that periodontal diseases and other oral diseases also affect viral infections. It is expected that future interdisciplinary studies on the cooperation between medical and other fields will investigate whether the concept of "periodontal medicine" is applicable to viral infections.
| 【Project 3】 | Elucidation of molecular mechanisms of viral latency and its reactivation involving epigenetic regulation. |
|---|
In the nuclei of eukaryotic cells, long genomic DNA tightly combines with histone and nonhistone proteins to form a dynamic polymer called chromatin. Chromatin is present in two main forms, called "euchromatin" and "heterochromatin". It has become increasingly clear that post-translational modification of DNA-associated histone proteins in chromatin plays an important role in gene expression. Notably, the histone modifications, acetylation and methylation of histone 3 and histone 4 play a central epigenetic role in the organization of chromatin domains and on?off regulation of gene expression (Fig. 5). It is now understood that epigenetics plays an important role not only in the regulation of gene expression but also in the pathogenesis of a broad range of diseases such as cancer and microbial infections. I have been studying about viral latency and its breakdown by histone acetylation and methylation (1).
- Ref;
- (1)J. Oral science (review), 2011.
Fig 5 Viral latency and its breakdown is mediated by HATs and HDACs.
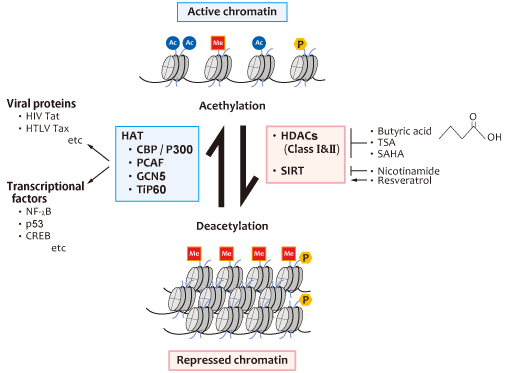
The dynamic equilibrium between histone Lys-acetylation catalyzed by histone acetyltransferases (HATs) and histone deacetylation catalyzed by histone deacetylases (HDACs) is crucial for on?off regulation of viral gene expression. Acetylation of the N-terminal tails of core histones by HATs (eg, CBP/p300) leads to open, transcriptionally active chromatin (viral gene expression: ON). HATs can also promote acetylation of nonhistone proteins, including many transcription factors and viral transactivators. Acetyl groups are removed by HDACs, the counterpart of HATs, which promote a closed, repressed chromatin structure (viral gene expression:OFF). SIRT is a class III HDAC, which promotes histone deacetylation by NAD-dependent mechanisms.
Mureaki Tamura-Antimicobial gel might prevent not only oral diseases but also systemic diseases.
Dental plaque is a product of microorganism accumulation and is a major pathogenic factor in the oral cavity. Mature dental plaque is known to cause both oral infections and systemic diseases. Therefore, the prevention of dental plaque formation, accumulation and maturation, which result from poor oral care, is important for the control of both oral and systemic diseases and requires the development of novel and more effective oral hygiene methods.
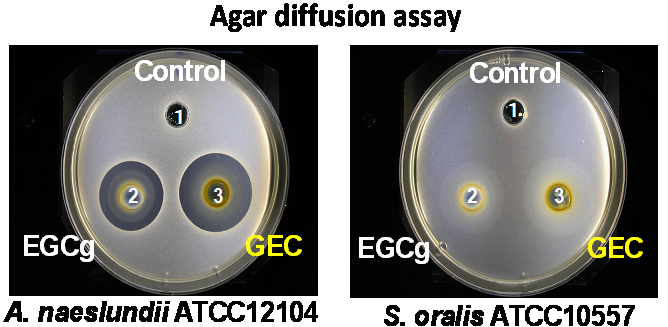
Catechin is polyphenolic chemical compound abundant in green tea and have been shown to exhibit physiological effects which include antibacterial, antifungal, antiviral, antioxidative, and antitumor activities. Recent studies have suggested that catechins help promote oral health and contribute to reducing risk of some systemic disease. However, the advantageous effects of catechins are not evident in the oral cavity as it serves simply as a passage for catechins and the use of catechin in oral care applications was uncommon. Our study aims to establish the efficacy of a catechin-gel mixture (catechin gel) incontrolling oral microbial populations and in preventing oral disease progression and systemic diseases (Fig. 1).
In addition, we also hope to clarify the anti-pathogenic activity of catechin against Candida albicans which is known to cause oral candidiasis, profound lung mycosis, aspiration of the digestive organs and, in the case of immunologically comprised states require long-term administration of antibacterial agents. In particular, we are elucidating whether catechin possesses anti-dimorphism activity by suppressing intracellular signal transduction (Fig. 2). Similarly, we are studying the development of an animal model that can be used to analyze the relationship between periodontal disease or oral candidiasis and systemic diseases (Fig. 3).
Fig 1
-
Fig 2
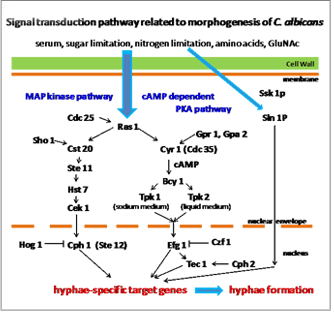
-
Fig 3
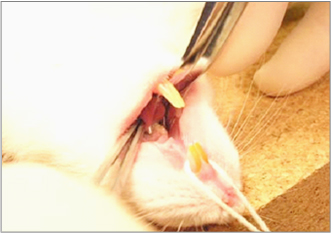
Noriaki Kamio -Relationship between periodontal disease and metabolic syndrome.
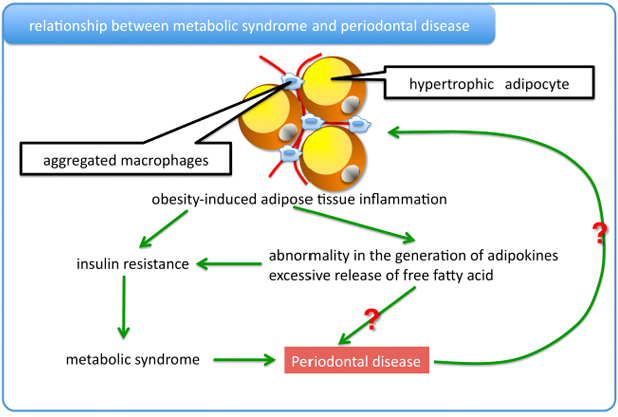
Relationship between periodontal disease and metabolic syndrome
An associtation between periodontal disease and metabolic syndrome based on cross-sectional and case-control studies was recently reported.
The recent study showed that individuals with periodontal pockets at baseline were more likely to develop components of metabolic syndrome and, moreover, the presence of periodontal pockets was associated with a positive conversion of metabolic syndrome components in a cohort study.
Adipocytes differentially release various proteins, so-called adipokines, and free fatty acids. Unbalanced production of pro- and anti-inflammatory adipokines seen in visceral fat obesity critically contributes to the development of many aspects of the metabolic syndrome. Saturated fatty acids, which are released from hypertrophied adipocytes, serve as an endogenous ligand for the TLR4.
In this regard, we are trying to uncover the molecular mechanism of the relationship between periodontal disease and metabolic syndrome.
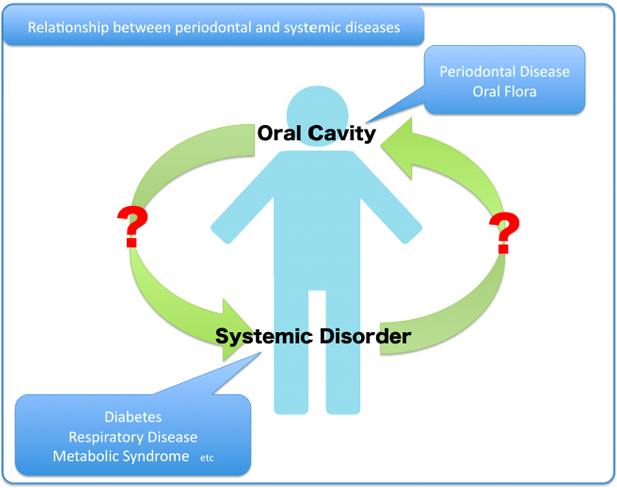
Relationship between periodontal and systemic disease
Periodontal diseases are a group of bacteria-associated inflammatory disease involving the supporting tissues of the teeth. In 1 mg of dental plaque, more than 100 million bacteria are present and over 700 species have been both identified and unidentified in these deposits. Normally, the dental plaque and the host immune response are in a balance which allows for periodontal health to be maintained, but pathogenicity can occur when the balance is compromised allowing a reduction in the proportion of beneficial bacteria and a deficit in the host immune system. On the basis of epidemiological studies, the association between chronic periodontitis and cardiovascular disease, respiratory disease, diabetes, osteoporosis, pre-term low birth weight and metabolic syndrome has been proposed.
We are trying to establish the relationship between periodontal disease and systemic disease.
Marni E. Cueno -Plant-based approach towards stress and vaccines.
Plants have been used in a variety of purposes which include but are not limited to food source and com mercial raw materials. My current work involves a combination science approach, whereby two or more unrelated fields of sciences are used to produce pioneering research work or provide a fresh approach,to wards understanding previously unexplained medical phenomena.
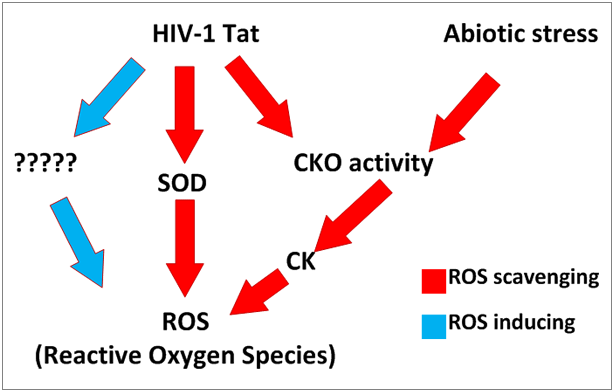
(1)Plant-based approach towards understanding HIV-1 Tat-induced oxidative stress
Plants have developed an extensive reactive oxygen species (ROS) scavenging system making plants a suitable host to test stress-inducing genes and proteins. HIV-1 Tat (transactivator of transcription) is known to cause oxidative stress by repressing superoxide dismutase (SOD) which subsequently allows ROS to increase. However, the mechanism behind Tat-induced ROS increase is still unknown. It is hoped that by expressing Tat in plants, a plant-based approach towards understanding the mechanism behind Tat-induced ROS increase can be elucidated.
Oral prophylactic vaccine production using plant cells
Several strategies have been used to make plant vaccines, in particular edible vaccines. A novel approach towards making plant-based vaccines would be with the use of a protein motif that would allow access of the leaderless secretory pathway. By allowing proteins to be secreted from plant cells and collected in a water-based medium, an antigen solution is easily produced which can be used to induce animmune response in the oral, pharyngeal and/or intestinal mucosal lining.
Publications
2014
Research paper
- 1)Imai K, Kamio N, Cueno ME, Saito Y, Inoue H, Saito I, Ochiai K (2014) Role of the histone H3 lysine 9 methyltransferase Suv39h1 in maintaining Epstein–Barr virus latency in B95-8 cells. FEBS Journal. in press.
- 2)Cueno ME, Imai K, Tamura M, Ochiai K(2014)Butyric acid-induced rat jugular blood cytosolic oxidative stress is associated with SIRT1 decrease. Cell Stress Chaperones. 19, 295-298.
2013
Review paper
- 1)Victriano AF, Imai k, Okamoto T (2013) Interaction between endogenous bacterial flora and latent HIV infection. Clin Vaccine Immunol 20, 773-779.
- 2)Imai K, Ochiai K (2013) Effect of microbial coinfection with HIV-1 and butyric acid-producing anaerobic bacteria on AIDS progression. J Oral Biosci 55, 55-60.
Research paper
- 1)Cueno ME, Imai K, Matsukawa N, Tsukahara T, Kurita-Ochiai T, Ochiai K(2013)Butyric acid retention in gingival tissue induces oxidative stress in jugular blood mitochondria. Cell Stress Chaperones. 18(5), 661-665.
- 2)Saito H, Tamura M, Imai K, Ishigami T, Ochiai K (2013) Catechin inhibits Candida albicans dimorphism by disrupting Cek1 phosphorylation and cAMP synthesis. Microb Pathog. 56, 16-20.
- 3)Cueno ME, Imai K, Okamoto T, Ochiai K (2013) Overlapping glycosylation sequon influences the glycosylation pattern of a chimeric protein expressed in tomato leaf and callus. J Biotechnol. 164(1), 9-12.
- 4)Kato A, Imai K, Ochiai K, Ogata Y (2013) Higher prevalence of Epstein-Barr virus DNA in deeper periodontal pockets of chronic periodontitis in Japanese patients. PLOS ONE 8, e71990.
- 5)Cueno ME, Imai K, Shimizu K, Ochiai K (2013) Homology modeling study toward identifying structural properties in the HA2 B-loop that would influence the HA1 receptor-binding site. J Mol Graph Model. 44, 161-167.
- 6)Yoneda S, Kawarai T, Narisawa N, Tuba EB, Sato N, Tsugane T, Saeki Y, Ochiai K, Senpuku H (2013) Effects of short-chain fatty acids on Actinomyces naeslundii biofilm formation. Mol Oral Microbiol 28, 354-365.
- 7)Cueno ME, Tamura M, Imai K, Ochiai K (2013) Orally supplemented catechin increases heme amounts and catalase activities in rat heart blood mitochondria: A comparison between middle-aged and young rats. Exp Gerontol. 48, 1319-1322.
- 8)Cueno ME, Imai K, Tamura M, Ochiai K (2013) Structural Differences between the Avian and Human H7N9 Hemagglutinin Proteins Are Attributable to Modifications in Salt Bridge Formation: A Computational Study with Implications in Viral Evolution. PLOS ONE. 8(10), e76764.
- 9)Kamio N, Kawato T, Tanabe N, Kitami S, Morita T, Ochiai K, Maeno M (2013) Vaspin attenuates RANKL-induced osteoclast formation in RAW264.7 cells. Connect Tissue Res. 54(2), 147-152.
- 10)Nakai K, Kawato T, Morita T, Iinuma T, Kamio N, Zhao N, Maeno M (2013) Angiotensin II induces the production of MMP-3 and MMP-13 through the MAPK signaling pathways via the AT1 receptor in osteoblasts. Biochimie 95, 922-933.
- 11)Tsuda H, Zhao N, Imai K, Ochiai K, Yang P, Suzuki N (2013) BIX01294 suppresses osteoclast differentiation on mouse macrophage-like Raw264.7 cells. Bosn J Basic Med Sci 13, 271-275.
- 12)Yonezawa H, Osaki T, Hanawa T, Kurata S, Ochiai K, Kamiya S (2013) Impact of Helicobacter pylori biofilm formation on clarithromycin susceptibility and generation of resistance mutations. PLOS ONE 8, e73301.
- 13)Cueno ME, Imai K, Ochiai K(2013)Structural insights on the potential significance of the twin Asn-residue found at the base of the hemagglutinin 2 stalk in all influenza A H1N1 strains: a computational study with clinical implications. OMICS. 17(6), 297-301.
- 14)Cueno ME, Imai K, Tamura M, Ochiai K (2013) Butyric acid-induced rat jugular blood cytosolic oxidative stress is associated with SIRT1 decrease.Cell Stress Chaperon (ahead of print)
2012
Review paper
- 1)Imai K, Ochiai K, Okamoto T (2012) Microbial interaction between HIV-1 and anaerobic bacteria producing butyric acid: its potential implication in AIDS progression. Future virology. in press.
- 2)Imai K, Ogata Y, Ochiai K (2012) Microbial interaction of periodontopathic bacteria and Epstein–Barr virus and their implication of periodontal diseases. J. Oral Biosci., 54(3), 164-168
- 3)Tamura M, Ochiai K (2012) Exploring the possible application of catechin (gel) for oral care of the elderly and disabled individuals. JDSR, 48(2), 126-134
- 4)Imai, k, Victriano, AF, Ochiai, K, Okamoto, T (2012) Microbial Interaction of Periodontopathic Bacterium Porphyromonas gingivalis and HIV -Possible Causal Link of Periodontal Diseases to AIDS Progression-Current HIV Research Vol.10, 238-244
Research paper
- 1)Nakai K, Kawato T, Morita T, Iinuma T, Kamio N, Zhao N, Maeno M (2012) Angiotensin II induces the production of MMP-3 and MMP-13 through the MAPK signaling pathways via the AT(1) receptor in osteoblasts. Biochimie. 2012 Dec 28.
- 2)Miyazaki Y, Inoue H, Kikuchi K, Ochiai K, Kusama K.(2012)Activation-induced cytidine deaminase mRNA expression in oral squamous cell carcinoma-derived cell lines is upregulated by inflammatory cytokines. J Oral Sci. 54(1), 71-75.
- 3)Imai K, Yamada K, Tamura M, Ochiai K, Okamoto T (2012) Reactivation of latent HIV-1 by a wide variety of butyric acid-producing bacteria.Cell Mol Life Sci. 69(15), 2583-2592
- 4)Cueno ME, Imai K, Ochiai K, Okamoto T (2012) Cytokinin dehydrogenase differentially regulates cytokinin and indirectly affects hydrogen peroxide accumulation in tomato leaf. J Plant Physiol. 169(8), 834-838
- 5)Imai K, Inoue, H, Tamura M, Cueno ME, Inoue H, Takeichi O, Kusama K, Saito, I, Ochiai K (2012) The periodontal pathogen Porphyromonas gingivalis induces the Epstein-Barr Virus lytic switch transactivator ZEBRA by histone modification. Biochimie 94(3), 839-846
- 6)Yonezawa H, Osaki T, Hanawa T, Kurata S, Zaman C, Woo TD, Takahashi M, Matsubara S, Kawakami H,Ochiai K, Kamiya S (2012) The destructive effects of butyrate on the cell envelope of Helicobacter pylori. J Med Microbiol. 61(Pt 4), 582-589
2011
Review paper
- 1)Ochiai K, Imai K, Tamura M, Ochiai-kurita T (2011) Butyric acid effects in the development of periodontitis and systemic diseases. J. Oral Biosci., 53(3), 213-220.
- 2)Imai K, Ochiai K. (2011) Role of histone modification on the transcriptional regulation and HIV-1 gene expression?Possible mechanisms of periodontal diseases in AIDS progression?. J. Oral science, 53(1), 1-13
Research paper
- 1)Tamura M, Saito H, Kikuchi K, Ishigami T, Toyama Y, Takami M, Ochiai K (2011) Antimicrobial Activity of Gel-Entrapped Catechins toward Oral Microorganisms. Biological & Pharmaceutical Bulletin 34(5),638-643
- 2)Zhang F, Tanaka H, Kawato T, Kitami S, Nakai K, Motohashi M, Suzuki N, Wang CL, Ochiai K , IsokawaK, Maeno M (2010) Interleukin-17A induces cathepsin K and MMP-9 expression in osteoclasts via celecoxib-blocked prostaglandin E-2 in osteoblasts. Biochimie 93(2), 296-305
- 3)Ogawa A, Furukawa S, Fujita S, Mitobe J, Kawarai T, Narisawa N, Sekizuka T, Kuroda M, Ochiai K, Ogihara H, Kosono S, Yoneda S, Watanabe H, Morinaga Y, Uematsu H, Senpuku H (2011) Inhibition of Streptococcus mutans Biofilm Formation by Streptococcus salivarius FruA. Applied and environmental microbiology 77(5), 1572-1580
- 4)Nakao R, Hasegawa H, Ochiai K, Takashiba S, Ainai A, Ohnishi M, Watanabe H, Senpuku H. (2011) Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS One 6(10), e26163.
- 5)Victoriano AF, Imai K, Togami H, Ueno T, Asamitsu K, Suzuki T, Miyata N, Ochiai K, Okamoto T (2011)Novel histone deacetylase inhibitor NCH-51 activates latent HIV-1 gene expression. FEBS. L. 685 (7), 1103-1111
- 6)Tanigawa S, Aida Y, Kawato T, Honda K, Nakayama G, Motohashi M, Suzuki N, Ochiai K, Matsumura H,Maeno M (2011) Interleukin-17F affects cartilage matrix turnover by increasing the expression of collagenases and stromelysin-1 and by decreasing the expression of their inhibitors and extracellular matrix components in chondrocytes. Cytokine 56(2), 376-386
- 7)Narisawa N, Kawarai T, Suzuki N, Sato Y, Ochiai K, Ohnishi M, Watanabe H, Senpuku H. Competence-dependent endogenous DNA rearrangement and uptake of extracellular DNA give a natural variant of Streptococcus mutans without biofilm formation. J Bacteriol. 2011 Oct;193(19):5147-54
- 8)Iida T, Kawato T, Tanaka H, Tanabe N, Nakai K, Zhao N, Suzuki N, Ochiai K, Maeno M (2011) Sodium butyrate induces the production of cyclooxygenases and prostaglandin E2 in ROS 17/2.8 osteoblastic cells. Arch Oral Biol. 56(7) , 678-686
- 9)Asamitsu K, Hibi Y, Imai K, Victoriano AF, Kurimoto E, Kato K, Okamoto T (2011) Functional characterization of human cyclin T1 N-terminal region for human immunodeficiency virus-1 Tat transcriptional activation. J Mol Biol., Vol. 410(5), 887-895.
- 10)Kamio N, Akifusa S, Yamashita Y (2011) Diacylglycerol kinase alpha regulates globular adiponectin-induced reactive oxygen species. Free Radic Res. 45(3),336-341
- 11)Mikami Y, Senoo M, Lee M, Yamada K, Ochiai K, Honda MJ, Watanabe E, Watanabe N, Somei M, Takagi M (2011) Inhibitory Effects of a Tryptamine Derivative on Ultraviolet Radiation-Induced Apoptosis in MC3T3-E1 Mouse Osteoblasts. J pharmacological science 115(2), 214-220
2010
Review paper
- 1)Imai, K, Okamoto, T, Ochiai, K. Molecular mechanisms of HIV-1 latency and its breakdown by Periodontal diseases. J. Oral Biosci. Vol. 52 (3): p.260-267, 2010.
Research paper
- 1)Ikeda, H, Miyatake, M, Koshikawa, N, Ochiai, K. Yamada, K, Kiss, A, Donlin, MJ, Panneton, WN, Churchill, JD, Green, M, Siddiqui, AM, Leinweber, AL, Crews, NR, Ezerskiy, LA, Rendell, VR, Belchev, MM,Coscia, CJ. Morphine modulation of thrombspondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J Biol Chem, 285(49), 38415-27
- 2)Jin M, Satsu H, Yamada K, Hisada N, Totsuka M, Shimizu M. Permeation of disaccharides derived from chondroitin sulfate through human intestinal Caco-2 cell monolayers via the paracellular pathway.Biosci Biotechnol Biochem 74, 1243-1249
- 3)Imai K, Togami H, Okamoto T. Involvement of histone H3 Lysine 9 (H3K9) methyl transferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. J Biol Chem. Vol. 285 (22): p.16538-16545
- 4)Jin M, Iwamoto T, Yamada K, Satsu H, Totsuka M, Shimizu M. Disaccharide derived from chondroitin sulfate A suppressed CpG-induced IL-6 secretion in macrophage-like J774.1 cells. Cytokine 51, 53-59
- 5)Tsuda H, Ochiai K, Suzuki N, Otsuka K. Butyrate, a bacterial metabolite, induces apoptosis and autophagic cell death in gingival epitherial cells. J. periodontal Res 45, 626-634
- 6)Kurita-Ochiai T, Ochiai K. Butyric acid induces apoptosis via oxidative stress in Jurkat T-cells. J Dent Res 89, 689-694
- 7)Cueno ME, Hibi Y, Imai K, Laurena AC, Okamoto T. Impaired plant growth and development caused by human immunodeficiency virus type 1 Tat. Transgenic Res. Vol. 19(5): p.903-913
- 8)Miyazaki Y, Kikuchi K, Gonzalez-Ala P, Inoue, H, Noguchi, Y, Tsuchiya, H, Hayashi, J, Shin, K, Ochiai K, Kusama K. Association of butyric acid produces by periodontopathic bacteria with progression of oral cancer. J Canc Sci Ther 2, 26-32, 2010.
- 9)Cueno ME, Hibi Y, Karamatsu K, Yasutomi Y, Imai K, Laurena AC, Okamoto T. Preferential expression and immunogenicity of HIV-1 Tat fusion protein expressed in tomato plant. Transgenic Res. Vol. 19(5): 889-895
- 10)Tsuchiya A, Imai K, Asamitsu K, Waguri-Nagaya Y, Otsuka T, Okamoto T. Inhibition of inflammatory cytokine production from rheumatoid synovial fibroblasts by a novel IkappaB kinase inhibitor. J Pharmacol Exp Ther. Vol.333(1): p.236-243
- 11)Kurita-Ochiai T, Ochiai K. Butyric acid induces apoptosis via oxidative stress in Jurkat T-cells. J Dent Res 89, 689-694
Education
Department of Microbiology at the Nihon University School of Dentistry has been administering more than 200 hours of lessons a year, which include lectures and laboratory work practices for dental students. Focus of these lessons includes microbiology, immunology and chemotherapeutics against microbial infections. Our staff also serves as tutors to some problem-solving lessons of other subjects, such as statistics, general biology and human health sciences. In addition, we also send our staff as lecturers to an affiliated training school for oral hygienists. Occasionally, we invite guest speakers to conduct specialized lectures done for dental students. We strive to prepare future dentists by familiarizing them with a thorough background of oral microbiology needed for the present-day dentists.
Access
- Address:
- Department of Microbiology, Nihon University School of Dentistry,
1-8-13 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-8310, Japan - Tel:
- +81-3-3219-8125
- Fax:
- +81-3-3219-8317